What Does a Coefficient Represent in a Chemical Formula
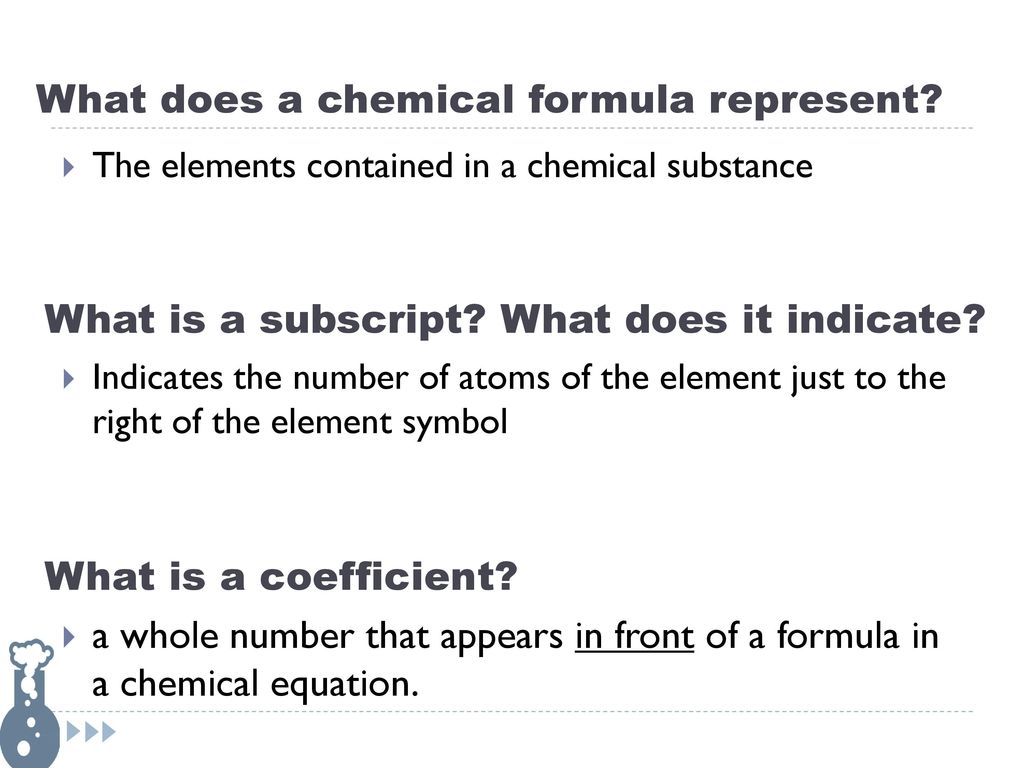
The purpose of coefficients in a chemical equation is that coefficients are used to show the ratio in which reactants combine and products for a chemical reaction. A coefficient is a number placed in front of a chemical symbol or formula.
Ch104 Chapter 5 Chemical Reactions Chemistry
Stoichiometric Coefficients This is known as the coefficient factor.

. We write this number in front of the substance when writing the chemical formula. The numerical coefficients in chemical equations show the ratio of the molecules of each substance involved in a chemical reaction both reactants and products. It shows how many atoms or molecules of the substance are involved in the reaction.
Coefficients are the numbers placed before the reactants in a chemical equation so that the number of atoms in the products on the right side of the equation are equal to the number of atoms in the reactants on the left side. Coefficients are numbers placed in front of a compoundmolecule in a chemical reaction. We know we have coefficients of.
The coefficient represents the number of atoms or molecules of the substance involved in the. A balanced chemical reaction means that the number of atoms of each element on both sides of the equation are the same. 1 for N 2.
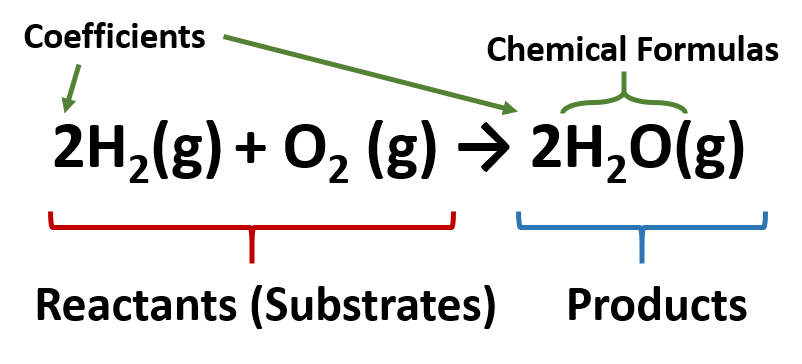
This tells us that two molecules of hydrogen H2 will react with one molecule of oxygen O2 to give two molecules of water H2O. 3 for H 2. What does a coefficient represent in chemical formula.
It shows how many atoms or molecules of the substance are involved in the reaction. In Chemistry the coefficient is the number in front of the formula. To do this you add coefficients as needed and these coefficients represent mole ratios of either reactants or products.
For every two unit of hydrogen molecule you need one unit of oxygen molecule and the result is two unit of water molecule. The coefficients are a ratio. It shows how many atoms or molecules of the substance are involved in the reaction.
A coefficient is a number that gives the number of moles of a substance that takes part in a particular chemical reaction. Very often in chemical formulae we use parentheses to form subgroups of atoms within a molecule. Be sure to answer all parts.
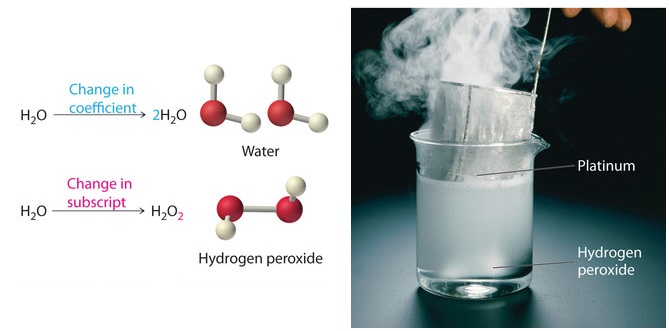
In such a formula the subscript outside the parentheses means that to count atoms you must multiply that subscript by the numbers of atoms inside. For example two molecules of hydrogen would be written as 2 H 2 and two molecules of water would be written 2. Moreover we can write the coefficient in the same size as other chemical symbols not as a subscript or as a superscript.
What do parentheses mean in a chemical equation. These are called the coefficients. A coefficient is a number placed in front of a chemical symbol or formula.
There are 4 chromium atoms and 6 oxygen atoms on the right side of the equation. In the balanced chemical equation for Ammonia. Here is the example equation again.
Coefficients are used to balance chemical equations. Why are coefficients important in stoichiometry. N 2 3H 2 2N H 3.
Coefficients are used to balance chemical equations. To balance chemical equations coefficients are used. A coefficient is a number placed in front of a chemical symbol or formula.
The number of molecules. It represents the amount of the substance. That unit is often the particle itself whether its an atom a metal ion a more substantial molecule or even a protein or enzyme.
Coefficients in a chemical equation represent the number of units of the formula immediately following the coefficient that are involved. It shows how many atoms or molecules of the substance are involved in the reaction. The coefficient tells us how many molecules of a given formula are present.
The amount of a substance is measured in moles. Coefficients are used to balance chemical equations. For example two molecules of.
In chemistry a coefficient in front of a chemical formula tells you how many moles you have. How many atoms of each type of element does this represent. It can mean the number of atoms molecules formula units or moles.
Both these terms refer to numbers but they give different details about a particular chemical reaction. 2H 2 O 2--- 2H 2 O. A coefficient is a number placed in front of a chemical symbol or formula.
One term in a balanced chemical equation contained the coefficient 3 in front of the formula Al2SO43. Note the presence of a two in front of the hydrogen and also the water. In Chemistry the coefficient is the number in front of the formula.
These numbers give two very important pieces of information about the equation. The coefficient in a chemical formula represents the amount of each chemical present. The key difference between coefficient and subscript is that coefficient gives the number of moles of a substance whereas subscript gives the number of atoms present in a molecule.
You must understand both in order to read and to use chemical equations successfully. When balancing a chemical equation the law of conservation of matter must be upheld. One term in a balanced chemical equation contained the coefficient 3 in front of the formula Al2SO43.
2H 2O means we have 2 molecules of water. Likeif taken the example of. 2H_2 O_2 2H_2O Here 2 before H means two hydrogen molecules and 2 before H_2O means two water molecules.
The coefficient tells us how many molecules of a given formula are present.
7 4 How To Write Balanced Chemical Equations Chemistry Libretexts

Finding Missing Coefficients Youtube

What Is A Chemical Equation Definition And Examples
Chapter 8 Chemical Reactions And Equations Ppt Download
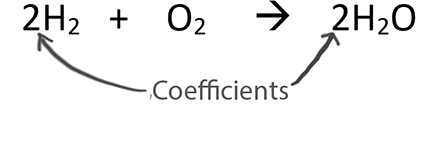
Breslyn Org Balancing Coefficients Subscripts

Do Now 1 What Is A Chemical Formula Give An Example 2 What Is A Chemical Equation 3 What Does The Law Of Conservation Of Mass Matter Say Agendatitle Ppt Download
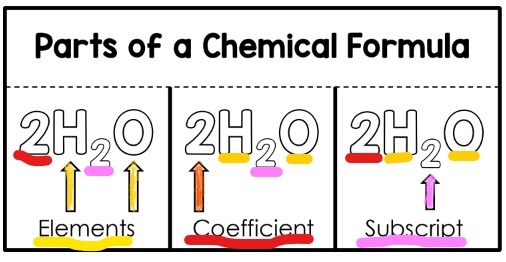
Subscripts Coefficients And Counting Atoms Quizizz
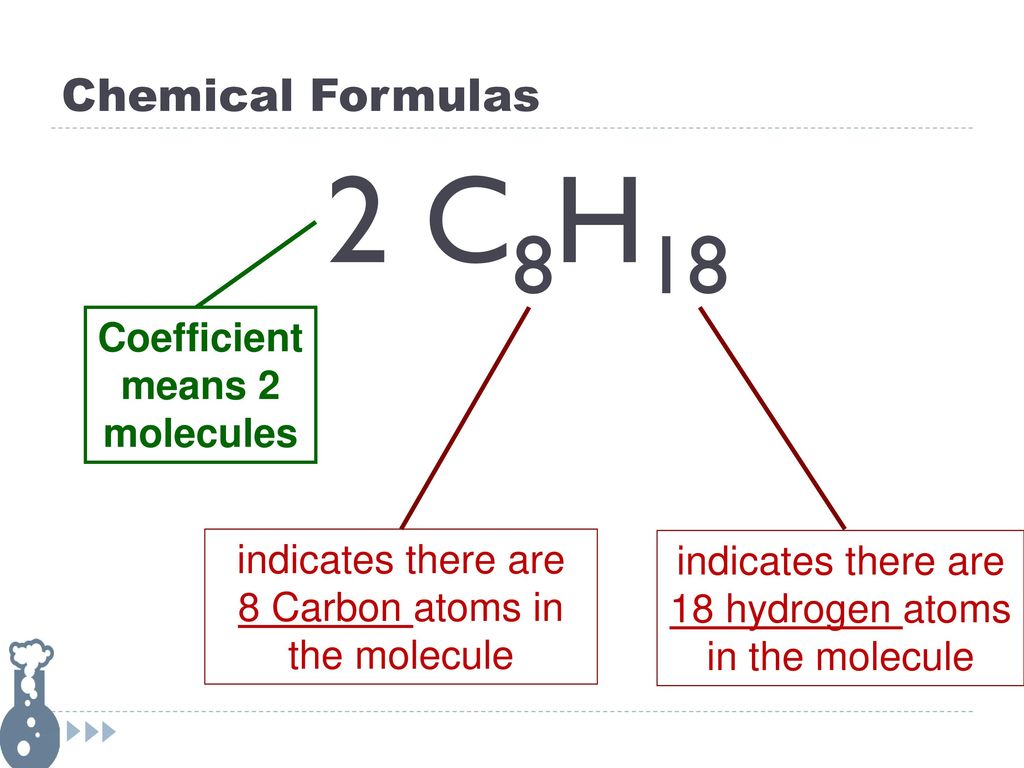
Chapter 8 Chemical Reactions And Equations Ppt Download
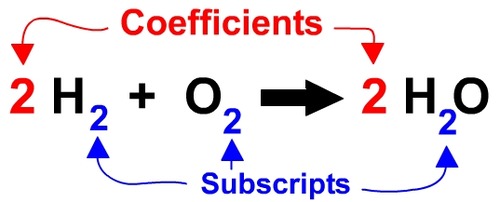
Subscripts Coefficients And Counting Atoms Quizizz
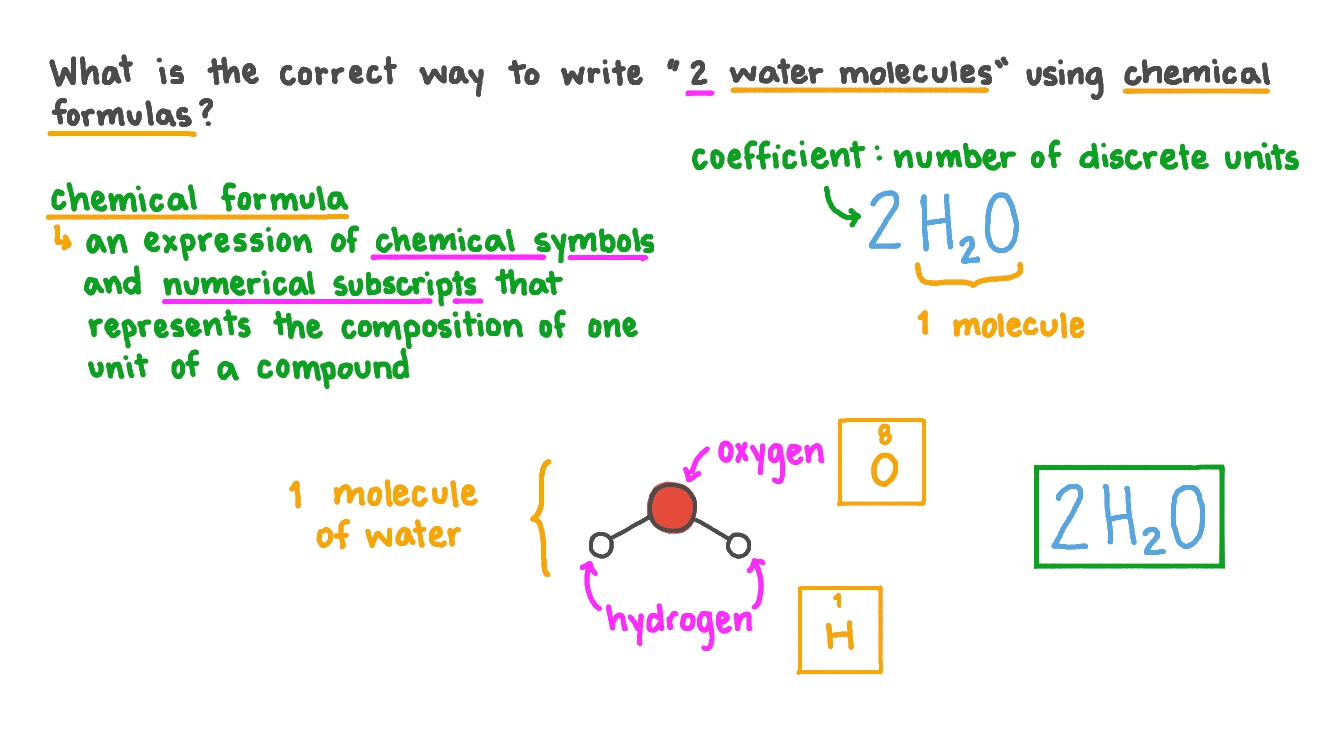
Question Video Expressing Numbers Of Molecules In Chemical Notation Nagwa

The Difference Between Coefficients And Subscripts In Chemical Equations Youtube

What Is A Chemical Equation Definition Examples Video Lesson Transcript Study Com

Balancing Chemical Equations Ck 12 Foundation

Writing And Balancing Chemical Equations Introductory Chemistry Lecture Lab

What Are Chemical Equations Detailed Explanation Examples

8 2 How Do We Represent Chemical Reactions Chemical Reactions Siyavula

Comments
Post a Comment